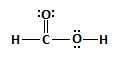VSEPR Theory Worksheet
Back to the other Bond Theory Chemistry Workbooks and other General Chemistry Workbooks
Go To -> Worksheet - Solutions Manual
- What is the premise of VSEPR theory?
- VSEPR Chart
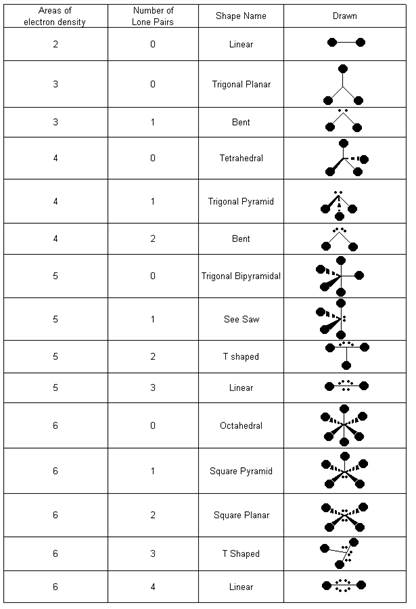
- How do electronic and molecular structure differ?
- What are the bond angles of the non-lone pair bearing shapes?
- Do lone pairs affect bond angles?
- How do you determine if a molecule is polar?
- What is a handy way to remember electronegativity ordering?
- How can shape affect polarity?
- Determine the molecular structure around each of the central atoms and whether the molecule is polar.
- CHF3
- I3-
- BrF5
- Explain why CF4 and XeF4 are non-polar yet SF4 is polar.
- Indicate the symmetry around all non-hydrogen atom